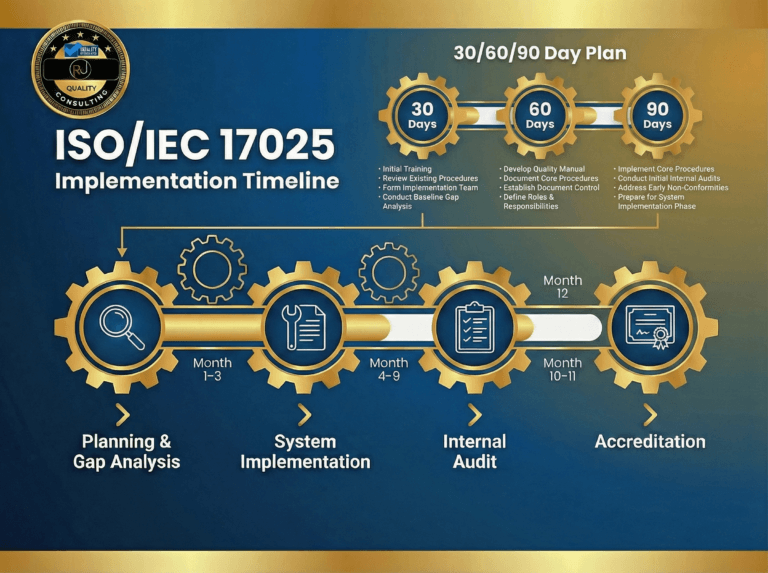
ISO 17025 Nonconformity Examples (Common Audit Findings Explained)
ISO/IEC 17025 audits can expose gaps fast—especially when a lab can’t show clear, traceable evidence for how work is performed and controlled. This guide provides practical ISO 17025 nonconformity examples and keeps common audit findings explained in plain English, so you can recognize what’s being cited, why it matters, and what objective evidence typically closes…