ISO 17025 Clauses 6 and 7: Technical vs Nontechnical Requirements Explained
Are you preparing your laboratory for ISO/IEC 17025:2017 accreditation? Understanding the ISO 17025 Clauses 6 and 7 of the standard is absolutely critical. These sections form the backbone of technical competence requirements for testing and calibration laboratories. In this post, we’ll break down what’s covered in Clauses 6 and 7, highlight the difference between technical and non-technical requirements, and share tips to help you get “audit ready.”
As part of my ISO/IEC 17025 clause-by-clause video blog series about the ISO 17025 clauses explained, this bonus video explores a critical but often overlooked distinction within Clauses 6 and 7 — the difference between technical and nontechnical requirements. As a certified ISO/IEC 17025 assessor, I break down which subclauses fall into each category, how they’re typically audited (lab-based vs. tabletop review), and why understanding this separation is essential for audit readiness and effective implementation of the standard.
Why Focus on ISO 17025 Clauses 6 and 7?
ISO/IEC 17025 Implementation Masterclass
Complete documentation + step-by-step training to get your lab accreditation-ready with confidence.
What you get (features)
- Customizable Quality Manual aligned to ISO/IEC 17025
- All required policies & procedures with matching forms/templates
- 7 training modules (Clauses 4–8, internal audit & management review)
- 20-question quiz + certificate of completion
- Clear instructions on tailoring documents to your lab
Why it matters (benefits)
- Implement faster with proven, audit-ready templates
- Train your team consistently and reduce nonconformities
- Show competence to assessors with documented training & certificate
- Confidently prepare for internal & external audits
- Move from “paper compliance” to a working quality system
ISO/IEC 17025:2017 is the international standard for laboratory competence. While the standard covers both management and technical requirements, ISO 17025 Clauses 6 and 7 are where most of the technical expectations reside. Understanding what auditors will look for in these sections—and how they’ll look for it—can make the difference between a smooth accreditation process and one filled with corrective actions.
How Do Auditors Assess ISO 17025 Clauses 6 and 7?
Accreditation audits are typically performed in two main ways:
- Tabletop Audits: The auditor reviews your documentation and procedures with the quality manager, often using a detailed checklist.
- Witness Audits: The auditor observes your analysts as they run actual test methods, verifying that procedures and records are followed in real time.
Both approaches are designed to verify that your laboratory meets the requirements of ISO/IEC 17025:2017—not just on paper, but in practice.
To better understand how accreditation bodies evaluate technical competence during audits — particularly the difference between witnessed testing and document-based reviews — you can refer to ILAC’s official guidance series on assessing laboratories, which includes helpful documents on both assessor training and audit methodology.
Technical Requirements in ISO 17025 Clauses 6 and 7
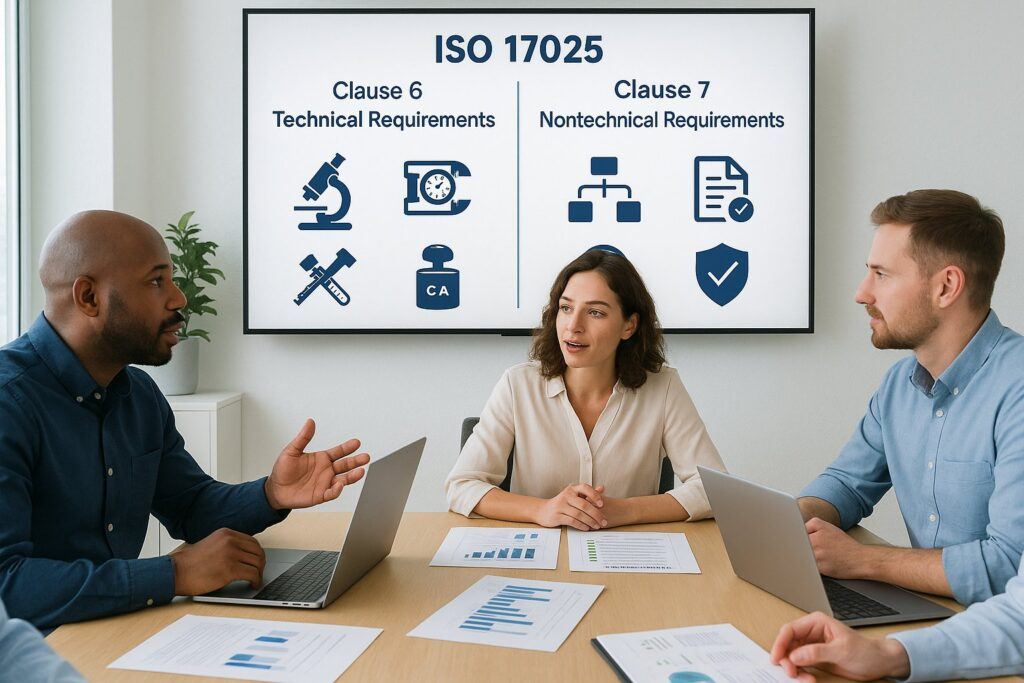
Clause 6: Resource Requirements
- 6.2 Personnel:
Auditors will check that staff are qualified and trained for their assigned tasks. Be prepared to present training records, job descriptions, and evidence of competency for each analyst. - 6.3 Facilities and Environmental Conditions:
Laboratories must monitor and record environmental conditions (such as temperature and humidity) as required by test methods. Auditors will want to see these records and understand your monitoring process. - 6.4 Equipment:
All equipment used in testing or calibration must be uniquely identified, properly labeled with calibration status, and tracked in maintenance logs. Equipment master lists and calibration certificates should be readily available. - 6.5 Metrological Traceability:
Calibration certificates for measuring equipment must be traceable to ISO/IEC 17025-accredited providers. If you use reference standards (e.g., CRMs), you’ll need certificates of analysis.
Clause 7: Process Requirements
- 7.2–7.8:
These sections cover the full lifecycle of test and calibration activities, including:- Selection and validation/verification of methodsSampling (where applicable)Handling and management of test items and samplesTechnical records (bench sheets, logs, LIMS entries)Measurement uncertainty estimationEnsuring validity of results through quality controlReporting of results to customers
Non-Technical Requirements in Clauses 6 & 7

While Clauses 6 and 7 are primarily technical, there are several non-technical requirements that are equally important for demonstrating laboratory competence and integrity.
- 6.1 General Resource Management:
Laboratories must ensure the availability of adequate resources (personnel, equipment, facilities, etc.). This is typically described in the quality manual. - 6.6 Externally Provided Products and Services:
You must have a procedure for evaluating and approving suppliers, maintain an approved vendors list, and keep records of supplier evaluations. - 7.1 Review of Requests, Tenders, and Contracts:
A documented procedure is required for reviewing customer requests and contracts, along with records of communications and contract reviews. - 7.9 Complaints:
Laboratories must have a complaint handling procedure and must log or record all complaints received. - 7.10 Nonconforming Work:
A procedure and records are needed for handling nonconforming work (e.g., out-of-spec results, process deviations). - 7.11 Control of Data and Information Management:
Any in-house LIMS or data management systems must be validated. All data must be secure and protected against unauthorized access or tampering, and system failures must be documented and escalated as needed.
Preparing for the Audit: Key Tips
- Document Everything:
Both technical and non-technical requirements rely on up-to-date, accessible documentation and records. - Practice Internal Audits:
Conduct your own tabletop and witness audits using the same checklists and methods auditors will use. - Train Your Team:
Ensure everyone understands their role in the quality system and can produce required records or explain procedures during an audit. - Review Your Quality Manual:
Make sure all required policies and procedures are in place and reflect your current practices. - Keep Records Organized:
Whether it’s training logs, equipment calibration certificates, or customer complaints, auditors will expect to see clear, traceable records.
Conclusion
Clauses 6 and 7 of ISO/IEC 17025:2017 are the heart of laboratory technical competence. By understanding and preparing for both the technical and non-technical requirements—and knowing how auditors will assess them—you can set your laboratory up for accreditation success.
If you need more detailed guidance, check out RJ Quality Consulting’s ISO 17025 Clauses Explained or consider booking a consultation with a qualified ISO/IEC 17025 assessor.
Ready to get accredited? Preparation is key!
If you found this post helpful, consider subscribing for more quality and accreditation tips, or reach out for a free consultation to discuss how we can help your lab succeed.
If you are interested in Going through the full Series, and/or you missed the other ISO 17025 Clauses walk-through video blogs, I have provided these resources for you to view.
| Clause | Topic | Link |
|---|---|---|
| Clause 4 | Impartiality & Confidentiality | ISO 17025 Clause 4 |
| Clause 5 | Structural Requirements | ISO 17025 Clause 5 |
| Clause 6 | Resource Requirements | ISO 17025 Clause 6 |
| Clause 7 | Process Requirements | Read Article |
| Clause 8 | Management System Requirements | Read Article |
| All | Laboratory Accreditation | How to become an ISO 17025 accredited laboratory |
🕒 Book Your Free 45-Minute Consultation
Have questions about ISO/IEC 17025 or ISO 9001 implementation or accreditation? Schedule a free 45-minute consultation with me to discuss your Company or laboratory’s needs and how we can achieve compliance together.
Schedule Your Consultation





