ISO 9001 Vs Iso 17025: Understanding the Overlap and the Gap
I often receive inquiries regarding quality and testing standards, especially concerning ISO 9001 vs ISO 17025. I have seen many organizations wrestle with understanding the features of these standards and with choosing which one suits their needs. In this article I share my experience and research to explain the key aspects of each standard, their differences, and what factors to consider if you plan to implement either or both frameworks.

Overview of ISO 9001 vs ISO 17025
My adventure in learning about quality and testing standards began when I first explored ISO 9001 and ISO 17025. In the early 2000’s I worked for a chemical manufacturing facility that was interested in ISO 9001, so they sent me to an ISO 9001 lead auditor training course provided by a very large registrar. Coincidently, I am now a certified assessor for the ISO/IEC 17025 standard by that same company. Because of this, I became very familiar with the similarities and differences between each of these well-known quality standards.
I learned that each standard supports organizations in different ways, and understanding their origins and purposes is a good starting point. ISO 9001 focuses on quality management systems while ISO 17025 lays out requirements for the competence of testing and calibration laboratories. This initial exploration helped me appreciate that both standards, despite their different focuses, aim to build a robust structure within organizations that truly supports excellence and reliability.
📘 ISO/IEC 17025 Quality Manual Template
Accelerate your lab’s accreditation process with our comprehensive Quality Manual Template, designed to align with ISO/IEC 17025:2017 standards.
- Fully editable and customizable to fit your laboratory’s needs.
- Includes all necessary procedures, forms, and policies.
- Structured to facilitate easy implementation and compliance.
- Developed by experts with extensive ISO/IEC 17025 experience.
Check Out Our Great Selection of ISO 9001 Online Training Courses
Looking to master ISO 9001:2015 or ensure top-notch training for your team? Explore our Online Courses here!
ISO 9001 is designed to help organizations meet customer requirements and step up satisfaction through a process-based approach. I have noticed that this standard is useful for any organization that wishes to demonstrate a commitment to quality and continuous improvement. Many businesses, regardless of industry, can benefit from adopting a quality management system that adheres to ISO 9001, ultimately fostering more transparent operations and better outcomes for customers.
On the other hand, ISO 17025 specifically targets testing and calibration laboratories. This standard helps labs show that they operate competently and produce valid results. I learned that laboratories seeking international recognition use ISO 17025 to build trust with their clients by confirming technical expertise and reliable measurement practices. It also establishes a framework that makes it easier for organizations to measure and maintain the accuracy of their outputs consistently.
Understanding ISO 9001

My initial exploration of ISO 9001 revealed that it is a flexible quality management standard applicable to a wide range of industries. Understanding the ISO 9001 standard is far easier than understanding ISO 17025. Organizations that adopt ISO 9001 generally aim to document processes, monitor performance, and drive continuous improvement. The structure of ISO 9001 makes it accessible to companies of all sizes, whether small businesses or large multinational corporations. Many managers have noted that having an all-in-one system in place helps reduce waste and streamlines internal communications.
The standard has a process-based approach that guides organizations in managing their internal procedures. This means that companies need to identify key processes, define measurable objectives, and ensure that roles and responsibilities are clear. I stumbled upon the fact that many organizations find value in this systematic approach because it lays the foundation for internal consistency and customer satisfaction. The clear and straightforward process mapping also aids in minimizing errors and ensuring that improvement is an ongoing activity rather than a one-time project.
Some key elements I noted include:
- Customer Focus: Emphasizes meeting customer requirements and striving to boost customer satisfaction.
- Process Approach: Involves understanding and managing interrelated processes as a system.
- Improvement and Innovation: Encourages organizations to continually improve their performance by assessing their processes and looking for innovative solutions.
This clear structure has made ISO 9001 especially attractive to organizations seeking to improve overall efficiency and product or service quality. Companies that adopt this standard often report streamlined operations, reduced inefficiencies, and more empowered teams who feel confident in the clarity of their tasks and roles.
Understanding ISO 17025

ISO 17025 is a standard that I regularly reference when discussing laboratory accreditation. It specifies the general requirements for the competence of testing and calibration laboratories. I appreciate that the ISO 17025 requirements focus on the technical operations and accuracy of laboratory testing, ensuring that every measurement or test result is reliable and reproducible.
Laboratories following ISO 17025 must demonstrate compliance with stringent procedures in aspects such as equipment calibration, method validation, and data traceability. My experience shows that this focus on precision and technical competence is very important in sectors such as environmental testing, clinical laboratories, and product certification. In fact, the rigorous nature of these requirements acts as a benchmark for many labs seeking improvement and international accreditation.
Speaking of ISO 17025 requirements, this video describes in detail the procedural and policy requirements of ISO 17025.
Important components of ISO 17025 include:
- Technical Competence: Involves ensuring that personnel have the necessary training and skills to perform tests and calibrations.
- Accuracy and Precision in Testing: Focuses on maintaining reliable and repeatable test outcomes with properly calibrated instruments.
- Quality of Results: Ensures that test results are valid and accepted by clients around the globe.
This approach provides laboratories with a framework to instill confidence in their testing outcomes. Laboratory staff, management, and regulatory bodies can all benefit from clearly documented protocols that facilitate a high standard of accuracy and clear accountability at every stage of the testing process.
Key Differences Between ISO 9001 and ISO 17025

When I first compared the two standards, the differences became immediately clear. ISO 9001 is a broad quality management standard that is not specific to any sector. It can be used for a manufacturing company just as well as a service-based company. Its flexibility allows it to be used across a multitude of processes and industries. The primary goal is to improve internal operations and ensure customer satisfaction through enhanced process control. In contrast, ISO 17025 is narrowly focused on laboratory competence, placing a strong emphasis on technical precision and standardization.
ISO 17025 is designed to ensure that testing and calibration labs deliver accurate and trustworthy data. Organizations that run labs might adopt both standards to cover overall quality systems and the precise technical competence needed in testing procedures. Choosing between these standards—or integrating them—depends on the nature of an organization’s operations and its primary objectives.
Here is a comparison of the two:
- Scope: ISO 9001 applies to overall quality management systems, while ISO 17025 is targeted at the operations of testing and calibration labs.
- Audience: ISO 9001 benefits a wide range of industries, whereas ISO 17025 is essential for labs that need to prove technical competence.
- Focus: ISO 9001 emphasizes customer satisfaction and process improvement. ISO 17025 focuses on technical competence, calibration of equipment, and test result reliability.
- Application: Many organizations implement ISO 9001 to standardize operations while laboratories may implement ISO 17025 to demonstrate their testing proficiency.
Understanding these differences helps organizations choose the standard that best serves their primary needs. I noticed that in some cases, organizations choose to integrate both standards for an all-in-one approach that covers both process efficiency and technical accuracy.
Implementation Challenges and Considerations
Implementing any standard requires a good understanding of the challenges involved. I have observed that organizations often face obstacles when seeking ISO certification. Planning and commitment to change are really important steps to ensure a successful transition. Preparing for certification means not only adjusting processes but also cultivating an organizational culture that values precision and accountability.
For ISO 9001, some common challenges include:
- Process Documentation: Documenting and mapping workflows can be time-consuming, even though it provides clarity and consistency in operations.
- Employee Training: Ensuring that all staff understand quality management concepts is a significant task. Continuous training helps maintain standards over time and makes the transition smoother for everyone involved.
- Resource Allocation: Implementing a thorough quality management system may require additional resources and strong management support to succeed in the long haul.
With ISO 17025, laboratory-specific challenges tend to revolve around:
- Technical Validation: Establishing validation protocols for testing methods requires detailed technical knowledge and meticulous documentation.
- Equipment Calibration: Maintaining calibrated instruments involves continuous monitoring and a strict schedule of maintenance that is essential for reliability.
- Data Integrity: Ensuring that test results remain accurate and traceable over time depends on robust data management practices that support both internal audits and external regulatory reviews.
In my experience, being proactive in addressing these challenges with a clear plan and involving all relevant stakeholders makes the transition smoother. It is helpful to seek advice from experts and consultation services experienced with ISO certifications. Beyond technical specifications, the human element in change management is equally important—communication, ongoing support, and real-time problem solving all contribute to achieving certification without overwhelming the organization.
Practical Guide to Achieving Certification
After understanding the differences and challenges, I often advise organizations to develop a practical plan if they decide to seek certification. Here are some steps I recommend to guide the process effectively:
- Conduct a Gap Analysis: Start by assessing your current procedures against the requirements of ISO 9001 or ISO 17025. This analysis reveals areas that need improvement and helps prioritize tasks.
- Develop a Roadmap: Based on the gap analysis, create a plan that outlines necessary changes, deadlines, and resource allocation. A clear roadmap provides direction and measurable benchmarks for progress.
- Get Involved Your Team: The success of the certification process relies on the active involvement of your entire team. Training and regular communication are critical to keep everyone informed and motivated.
- Implement and Monitor: As you start making changes, establish a system to monitor progress. Regular internal audits and performance reviews allow for timely adjustments along the way.
- Seek Certification from a Recognized Body: When you are confident that your system meets the standard, engage with an accredited certification body for the final audit and certification.
These steps have proven useful in my professional adventure, laying out a clear and manageable path toward achieving certification. The process not only reinforces the integrity of daily operations but also empowers the organization to maintain these high standards continuously.
Case Studies and Real-World Applications
To further illustrate the benefits of both ISO 9001 and ISO 17025, it is helpful to look at real-world examples. Many organizations have shared their success stories, explaining how adopting these standards transformed their processes through continuous improvement efforts. For instance, a mid-sized manufacturing company implemented ISO 9001 and experienced significant reductions in production errors and waste. By streamlining their workflow and documenting every process meticulously, they were able to maintain consistent quality even as they scaled operations. This is because the documented process was easier to train newer employees as the company expanded and increased their work force.
Similarly, a clinical laboratory that adopted the ISO 17025 documentation requirements reported not only higher accuracy in test results but also faster turnaround times. The rigorous documentation and regular reviews helped the lab identify bottlenecks early, leading to proactive maintenance of both equipment and protocols. Such case studies serve as a reminder that while the journey to certification can be challenging, the long-term benefits make the effort worthwhile. These real-life examples also show that successful implementation often requires strong leadership, a willingness to adjust, and continuous commitment from every level of the organization.
Advantages of a Laboratory Combining ISO 17025 with ISO 9001
Organizations that choose to combine both standards often find that the benefits are even more pronounced. For example, one calibration laboratory I worked with was able to adopt the principles of using measurable ISO 9001 quality objectives to drive improvement. This is something that is more prevalent in ISO 9001, yet when applied to the calibration services under ISO 17025 They not only optimize their internal processes but also build strong reputations in their respective industries for reliability and quality. This dual adoption can act as a powerful strategic tool, setting the stage for further growth and innovation.
The ISO/IEC 17025 discussed objective within section 8.2 under the ISO 17025 Management System Requirements of the standard and then again as a required input to Management Review in section 8.9, however, ISO 9001 captures quality objectives in more details and requires that the quality objectives be measurable and to be used to drive improvement. It has been my experience, that laboratories that use this approach to more easily find specific areas within the lab that they can improve upon and have seen the drive for improvement in a more prevalent fashion. I cannot emphasize the importance of understanding how establishing measurable objectives and keeping on top of them can drive improvement and even increases revenue.
To further elaborate on this point, below is an example of a Quality Objectives and KPI Register that a company can use to measure quality objectives and then discuss these objectives during a Management Review Meeting to brainstorm with involved personnel to come of with ideas to drive improvement.
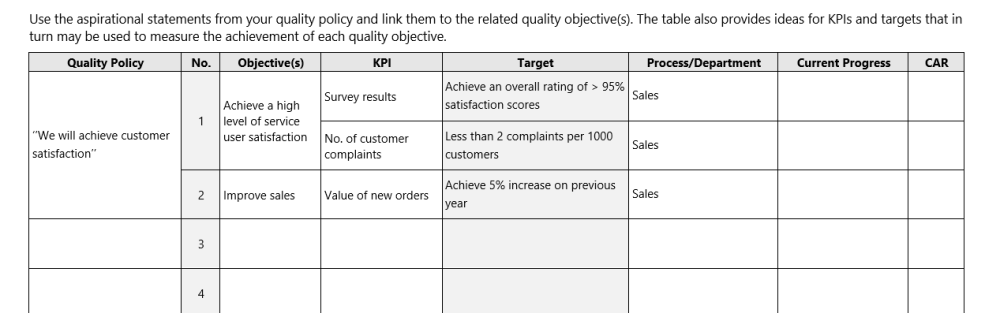
Advanced Insights and Best Practices
Over time, I have observed that continuous improvement is a recurring theme in both ISO 9001 and ISO 17025. Keep in mind, both standards are aligned with the International Organization for Standardization. Otherwise known as ISO. Not only is achieving certification important but maintaining and slowly stepping up the system is equally vital. Many organizations benefit from adopting best practices that can further boost their operations. Some advanced insights I have learned include:
- Integration of Systems: Organizations with multiple operational areas may choose to integrate ISO 9001 and ISO 17025. This approach simplifies management processes and helps preserve consistency across departments.
- Regular Training and Development: Continuous education is key. Ongoing training programs for employees keep systems fresh and ensure compliance with updated requirements. This also allows teams to adapt efficiently when industry standards evolve.
- Data-Driven Decision Making: By analyzing trends and performance metrics collected through these standards, organizations can make informed decisions that drive future improvements. This practice can lead to innovations in how processes are managed and refined over time.
- Commitment to Continuous Improvement: Both standards emphasize iterative progress. I have observed that establishing a culture of ongoing enhancement leads to sustainable long-term results and builds a foundation of trust with clients and regulatory agencies alike.
Adopting these practices has helped many organizations I have worked with to maximize the benefits of their certifications. It is not just about implementing a standard; it is about building an ecosystem where every team member is dedicated to quality and precision. Regular reviews, open communication, and a willingness to adjust along the way can turn initial certification into a lasting competitive advantage.
Frequently Asked Questions
I often get questions about the differences between these two standards. Here are some that come up frequently:
Question: What industries can benefit the most from ISO 9001 and ISO 17025?
Answer: ISO 9001 is useful for a wide range of industries including manufacturing, services, and retail. ISO 17025 is essential for laboratories, especially those involved in environmental testing and calibration services.
Question: Can an organization implement both ISO 9001 and ISO 17025?
Answer: Yes, many organizations with laboratories find it beneficial to integrate both. While ISO 9001 covers overall quality management, ISO 17025 ensures technical competence in laboratory operations.
Question: How long does the certification process typically take?
Answer: The time to achieve certification varies depending on the size and complexity of the organization. However, thorough preparation, detailed gap analysis, and ongoing training can help speed things up significantly.
Question: What are ongoing requirements after certification?
Answer: Regular audits, continual staff training, and a commitment to revising and improving procedures are necessary to maintain certification over time.
Final Thoughts
Deciding between ISO 9001 and ISO 17025, or even integrating both standards, depends on your organization’s specific needs and goals. I have found that taking the time to understand each standard offers significant benefits whether you are aiming to improve your quality management system or ensure laboratory competency. Investing in these standards not only demonstrates a commitment to quality and technical excellence but also lays the groundwork for better operational control and improved customer satisfaction.
Organizations that successfully implement these standards report not only improved compliance and operational efficiencies but also higher confidence from customers and regulatory bodies alike. It is important to research further, carefully map out your plans, and ensure that all team members are fully on board. By doing so, you build a foundation of trust and reliability that can make a lasting difference in achieving your business goals.
Thank you for reading this detailed comparison and guide. I hope the information shared here helps you make a more informed decision regarding quality management and laboratory competence certifications. Your dedication to improvement and clear process strategies can truly make a difference in driving long-term success for your organization.
🕒 Book Your Free 45-Minute Consultation
Have questions about ISO/IEC 17025 or ISO 9001 implementation or accreditation? Schedule a free 45-minute consultation with me to discuss your Company or laboratory’s needs and how we can achieve compliance together.
Schedule Your Consultation





