Ensuring the Validity of Results ISO 17025: Strategies for Compliant Laboratories
When it comes to laboratory testing, the significance of ensuring the validity of results ISO 17025 cannot be overstated. It’s an assurance of precision that supports trust in laboratory results, providing an international benchmark for accuracy and reliability. Think of it not just as a mere checklist but as a foundation for creating results that stakeholders can depend on. Laboratories worldwide turn to this standard as a testament to their commitment to excellence, demonstrating that their findings are not only consistent but also stand up to international scrutiny.
By adhering to the guidelines of ISO/IEC 17025:2017, specifically Section 7.7, laboratories take on a rigorous process of validation to ensure the integrity of their test results. This crucial step is not just about performing routine tests; it’s about ensuring those tests yield unquestionable evidence of their claims. This verification process is integral, not just for upholding the laboratory’s professionalism, but also for instilling a worldwide confidence across industries that depend on accurate lab data.
Key Takeaways
- ISO/IEC 17025:2017 is a cornerstone for establishing trust in laboratory data through accuracy and reliability.
- Section 7.7 of the standard provides a structured approach to consistently produce verifiable and solid test results.
- This commitment to validity is fundamental for a laboratory’s credibility and for maintaining confidence amid the global scientific community.
Ensuring the Validity of Results ISO 17025 and Developing Strategies for Laboratory Precision and Consistency

To produce results of impeccable accuracy and dependability in a laboratory setting, establishing comprehensive strategies for ensuring the validity of results ISO 17025 is essential. ISO/IEC 17025:2017 lays the groundwork for a quality framework that enhances every facet of laboratory functionality. Here’s how you can apply this to your operations:
Recording Data Meticulously: Begin by ensuring all data is logged with precision. This meticulous recording acts as a shield against inaccuracies and inconsistencies.
Book a FREE 45-Minute Consultation
During the Consultation We Will Give You a Clear Direction and Path For You to Move Forward with Your Certification or Accreditation Goals
- Utilize High-Quality Benchmarks:
- Quality Control Materials: Select these materials wisely to align with your testing requirements.
- Reference Standards: Use these to establish a baseline for data evaluation, strengthening the consistency of results.
Regular Equipment Evaluation:
- Daily Checks: Implement daily inspections of lab equipment, similar to pre-operation checks in other industries.
- Replicate Testing: Introduce duplicate assessments for a robust comparison.
- Intermediate Checks: These additional evaluations further reinforce result accuracy.
Training and Competency:
- Technician Proficiency: Confirm that technicians and analysts possess the necessary skills to carry out measurements effectively.
- Continuous Training: Ongoing education ensures that all personnel remain up-to-date with the latest procedures and technologies.
Calibration and Methodology:
- Calibration Practices: Employ consistent calibration of instruments to uphold testing accuracy.
- Documented Methods: Maintain clearly outlined and accessible processes for every test conducted.
- Audit Trails: Ensure that each testing sequence is accompanied by a traceable record.
- Reliability and Retesting: Build into your process the routine verification of results, ready to retest if necessary.
By weaving these practices into the fabric of your laboratory’s daily routine, you not only commit to the ethos of ISO/IEC 17025 but also to delivering reliable and quality results you and your clients can trust.
Ensuring the Validity of Results ISO 17025 through Proficiency Testing and Collaborative Comparisons

- ISO 17025 Proficiency Testing (PT) and Collaborative Laboratory Comparisons (CLC) are pivotal in maintaining the integrity of laboratory data.
- Engaging in these evaluations provides an objective review, ensuring your laboratory methodologies and equipment yield precise results.
- Through PT/CLC, your laboratory’s performance is measured against peers, enhancing trust among clients.
It’s imperative for your laboratory to regularly participate in PT/CLC, aligning with the expectations of ISO 17025, particularly section 7.7. This participation isn’t merely a procedural formality but a vital practice to verify that testing methods remain suitable for their intended purposes. Regardless of whether you’re assessing the chemical balance of aquatic environments or the robustness of construction materials, PA/CLC furnishes a methodical way to corroborate the efficacy of your testing procedures. By consistently engaging in such comparative analyses, you fortify the credibility of your laboratory’s outputs.
The Role of ISO 17025 Management Review in Proficiency Testing
ISO/IEC 17025 mandates that during the management review process, you must examine and evaluate your proficiency testing procedures and have a section that is specific to ensuring the validity of results ISO 17025. This vital part of the standard ensures that your laboratory’s Quality Management System is not only compliant but also fosters continual improvement and competence.
- Risk Management: Building a proficiency testing plan should be a systematic process grounded in effective risk management. This strategy ensures that your procedures are neither random nor haphazard, but are instead a fit to your laboratory’s specific demands.
- Technician Competence: Proficiency testing serves as a measure of your technicians’ skills. High performance indicates proficient laboratory practices, while poor results may point out areas where additional training may be beneficial.
- Strategic Planning: In the event that external proficiency testing services are unavailable, it’s essential to refer to guidelines such as those detailed in ILAC P9 for creating an internal proficiency testing plan that aligns with your lab’s unique requirements.
- Documentation and Continuity: The management review should include a comprehensive plan covering a four-year cycle. This plan must encompass all key areas of your lab’s operational scope and engage all skilled personnel.
Remember, proficiency testing is about more than ticking a box for accreditation purposes—it’s about upholding a system that consistently ensures accurate results, and this calls for cooperation and a commitment to ongoing quality enhancement.
Analyzing Data and Improving Laboratory Practices
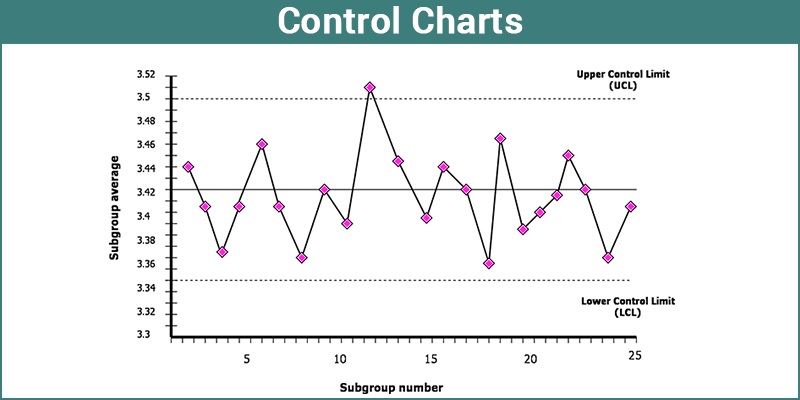
Laboratories dedicated to robust quality assurance polish their procedures and control their data by meticulously examining data from proficiency testing, interlaboratory comparisons and by the use of control charts internally. By employing statistical techniques and control charts, you can uncover patterns in your data, assessing trends and precision. These tools facilitate a deep dive into performance, pinpointing when test results deviate from established standards.
Here’s how you can harness these insights:
- Utilize control charts to monitor consistency and fluctuation in test outcomes, spotting any troubling trends that emerge by employing your ISO 17025 nonconforming work process.
- Conduct replicate testing using reference materials to validate the repeatability and reliability of your results.
- When data present anomalies, engage in a thorough evaluation. These irregularities can be informative, prompting you to take necessary action that will bolster confidence in your lab’s work.
If you analyze the image above, you will notice that there is a data point that is above the upper control limit (UCL). A laboratory that follows the requirements of ISO/IEC 17025 will create a nonconformance and address the issue even if it has a very simple solution associated with it. By having this documentation available for trending, the quality manager can assess trends in the data and take the necessary actions to address the root cause of the problem if there happens to be a common trend. This is an example of good quality control being applied.
Data analysis is not just about compliance; it’s about engraining a culture of excellence by continuously refining your methods. Here are some steps to ensure diligence:
- Analyze – Systematically review the collected data.
- Detect trends – Use charts to identify any changes or patterns.
- Evaluate risks – Consider the impact of odd data points on overall validity.
- Respond – Implement corrective actions swiftly to handle any irregularities.
Remember, addressing variations isn’t a setback but a step toward enhancing both your technique and the integrity of your results. Your adaptive strategies signal a commitment to providing trustworthy data. Keep refining your practices, as the aim is to prevent the delivery of inaccurate results—upon which the foundation of trust in scientific research is built.
Common Inquiries for ensuring the validity of results ISO 17025
Validation Requirements in ISO 17025
To comply with ISO 17025, your laboratory’s method validation should include a clear plan that details the following:
- Accuracy
- Precision
- Selectivity
- Linearity
- Range
- Detection limits
- Quantitation limits
- Robustness
Adhering to Clause 7.7 for Result Validity
To align with Clause 7.7 of ISO 17025, your lab is expected to undertake these actions:
- Record Keeping: Maintain detailed records of all data.
- Trend Detection: Monitor for and document any trends or anomalies.
- Reference Materials: Employ quality control materials for each sample set.
- Quality Control Checks: Assure regular QC checks with each batch.
Validation Process Review Frequency
Your validation processes should be reviewed:
- At planned intervals
- Whenever significant changes occur in the methodology
- In case of an equipment upgrade or replacement
Implications of Invalidation in ISO 17025 Labs
Ignoring result validity in your lab can lead to:
- Questioned credibility of generated results
- Loss of accreditation status
- Legal implications and loss of customer trust
Conclusion
To effectively ensure the validity of results in compliance with ISO 17025, laboratories must adopt rigorous validation processes and quality controls. This blog post has discussed the importance of systematic approaches, such as proper documentation, regular equipment maintenance, and adherence to standard operating procedures, to maintain the reliability and accuracy of test outcomes. These steps are not only essential for meeting ISO standards but also critical in building and sustaining the confidence of clients and stakeholders in laboratory results.
Also, the utilization of control charts to help ensure the validity of results ISO 17025 plays a pivotal role in maintaining the consistency and reliability of laboratory results. By implementing these charts, laboratories can monitor ongoing processes and immediately detect any deviations from the norm, facilitating timely corrective actions. This proactive approach not only supports compliance with ISO 17025 but also enhances the overall quality management system, ensuring that the test results are both accurate and reliable over time.






